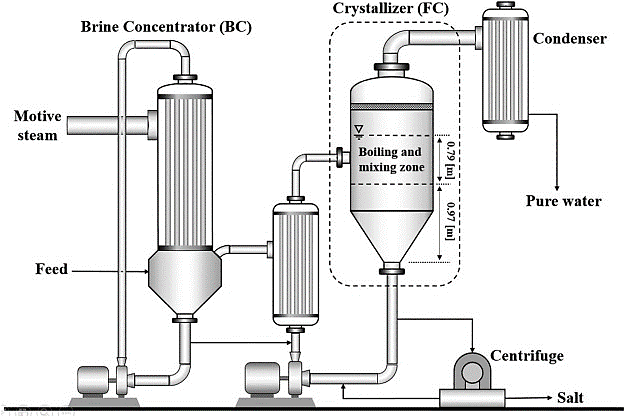
Crystallization is a physical change. Crystallization is the formation of solids from the liquid or gaseous phase. This technique includes obtaining the crystals of a soluble substance from a hot saturated solution and separating the soluble solid from the solution.
To concentrate feed into solid crystals and clean water, crystallizers are used. A progressively harder method for which crystallite sizes are formed from a liquid solution is known as crystallization. Crystallizers can remove liquid wastes completely, resulting in no liquid discharge (ZLD). The process continues and secondary crystallization are the two stages of crystallization. The formation of new crystals is referred to as primary nucleation. Secondary nucleation is the primary stage that results in the mass production of crystals. There are two types of crystallization processes: evaporative crystallization and cooling crystallization.
Be aware that from the initial situation of 2016, this journal works by using posting quantities instead of page figures. See even further facts in this article.
: Zero liquid discharge (ZLD) is a technique for treating higher-salinity brine to obtain freshwater and/or salt employing a photo voltaic interface evaporator. Even so, salt accumulation to the floor in the evaporator is a large challenge to preserving steady h2o evaporation. During this review, an easy and straightforward-to-manufacture evaporator, also known as a crystallizer, was created and fabricated by 3D printing. The photothermal layer printed with polylactic acid/carbon composites experienced suitable light absorption (93%) within the wavelength zone of 250 nm–2500 nm. The micron-sized voids shaped in the course of 3D printing provided considerable drinking water transportation channels In the crystallizer.
In 2016, Mi et al.37 regarded the salt accumulation to the photothermal material as a chance to obtain ZLD desalination in the photo voltaic nevertheless set up. When fifteen wt% pure NaCl aqueous brine was employed as supply h2o, a thick layer of accumulated NaCl crystals formed along with their two dimensional (second) graphene oxide (GO) membrane. The white salt layer substantially diminished light-weight absorption in the system, and triggered a considerable reduction in evaporation charge. On the other hand, a stable water evaporation charge of 0.five kg m−2 h−1 was however recorded, indicating the salt precipitation didn't block the evaporation. In 2018, A 3 dimensional (3D) cup-formed photo voltaic evaporator which divided The sunshine adsorption floor within the salt precipitation surface was noted.
As We all know, the evaporator is additionally appropriate for salt recovery from high-salinity brine. However, it's very effortless for salt to crystallize around the evaporator floor and inside the h2o channel although managing higher-salinity brine. The performance of photothermal conversion as well as the water transportation efficiency of evaporators will minimize on account of salt accumulation [22,23]. Consequently, enabling an evaporator to possess strong water evaporation effectiveness is essential to research all through salt recovery.
Gradual cooling: It necessitates solvents with lower boiling factors and modest solute solubility. A particular amount of money of fabric is required.
So that you can verify the system of forming the dense salt crust levels and interior pore filling crystals in the case of the real seawater brines, the crystallization behaviors of pure NaCl brine and concentrated SWRO brine have been investigated and as opposed (the experimental particulars are available in SI). The SEM photos with the crystals fashioned by evaporating twenty wt% pure NaCl brine (Fig. S14) clearly show person and properly divided cubic crystals only. For pure NaCl brine, as drinking water is staying faraway from the evaporation area, the temperature is decreased and the salt focus is increased on the surface, that makes the salt crystals usually precipitate over the outer area and as a consequence it retains the drinking water channel inside the QGF membrane unblocked.
The dense salt crust area layer along with the salt crystals filling Within the QGF membrane in the case in the concentrated SWRO brine leads to the failure in the 3D crystallizer all through the long term operations.
S5a). In these scenarios, in the event the water evaporation charges had dropped to almost zero, these crystallizer surfaces appeared fairly black, indicating The sunshine absorption of those crystallizers wasn't drastically degraded with the precipitated salt layer (Fig. S5b, c). As a result, it can be believed that the variation in the light absorption wasn't the true reason for the degraded water evaporation fees via the 3D crystallizer when dealing with the real brines.
As pointed out over, the h2o evaporation performances of the exact same solar crystallizer is Crystallizer Manufacturer Crystallizer For Zero Liquid Discharge System completely unique when it really is utilized to take care of pure NaCl brines and true seawater brines. In the former case, the h2o evaporation retains stable for a long time period, although it drops speedily inside the latter case, which happens to be ascribed to the large difference in salt crust constructions in The 2 situations.
Many crystals are formed when crystallizers are melted. Nearly all the solution or soften (usually > ninety%) crystallizes in suspension or with a cooled floor. The very little quantity of uncrystallized mother liquor retains impurities.
MDPI and/or maybe the editor(s) disclaim responsibility for almost any personal injury to folks or residence ensuing from any ideas, procedures, instructions or merchandise referred to during the content material.
S13), indicating the re-dissolution of the salt crystals was insignificant. In cases like this, we think the salt re-dissolution was slowed down via the drinking water evaporation in darkness, which limited the salt ions again diffusion from your evaporation substrate towards the resource brine. Upon turning on the light, the evaporation level was recovered to the exact same level as being the earlier. So, the 3D photo voltaic crystallizer can operate repeatedly in the course of working day and night time with no special care when treating the concentrated SWRO brine, along with the solid salts could be consistently removed from the unit.
Due to hydrophobicity of PLA, the 3D-printed parts have been subjected to hydrophilic modification. Given that the slope of the photothermal conversion layer would be the very important element that decides the helpful photothermal conversion region, four forms of crystallizers were being built, Together with the photothermal layer made up of angles of thirty°, 60°, 90°, and a hundred and twenty°, respectively. To guage the salt crystallization general performance of the crystallizers, the salt crystallization approach was operate less than an Intense salt concentration ecosystem (20 wt%). The experimental final results showed that the micron-sized water transport channel and superhydrophilicity from the crystallizer made constant drinking water evaporation and ideal salt crystallization effectiveness.
Take note: In Fig. 3c, an optional transparent polycarbonate defend with a peak of thirty mm was added on the photo voltaic crystallizer to be able to protect against salt crystal from creeping in to the internal facet of the crystallizer.